Atomic models of human tau filaments and development of tau ligands
Protein inclusions define many neurodegenerative diseases such as Alzheimer’s disease (AD) at the pathological level. Tau (MAPT) can aggregate to form multiple types of filaments. All six isoforms of tau are present in disease filaments as either paired helical (PFH) or straight (SF), with both types of filaments sharing a common structural subunit. Interestingly, the morphologies of tau filaments in different diseases vary, even when they consist of the same isoforms. Together with our collaborators, we elucidate the structural basis and pathological mechanisms implicated in sporadic, hereditary and secondary tauopathies by determining the atomic structure of tau filaments from the brain tissues of patients by cryo-EM and cryo-ET. With atomic models of tau filaments generated by cryoEM we can also identify specific ligands of tau filaments that can be used for in vivo imaging of patient brain regions that contain the filaments.
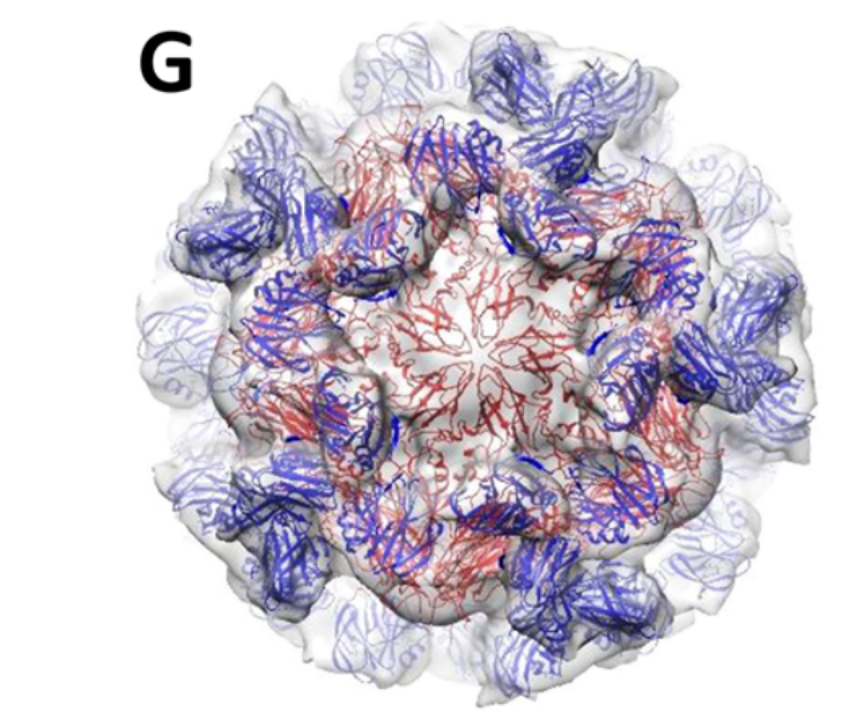
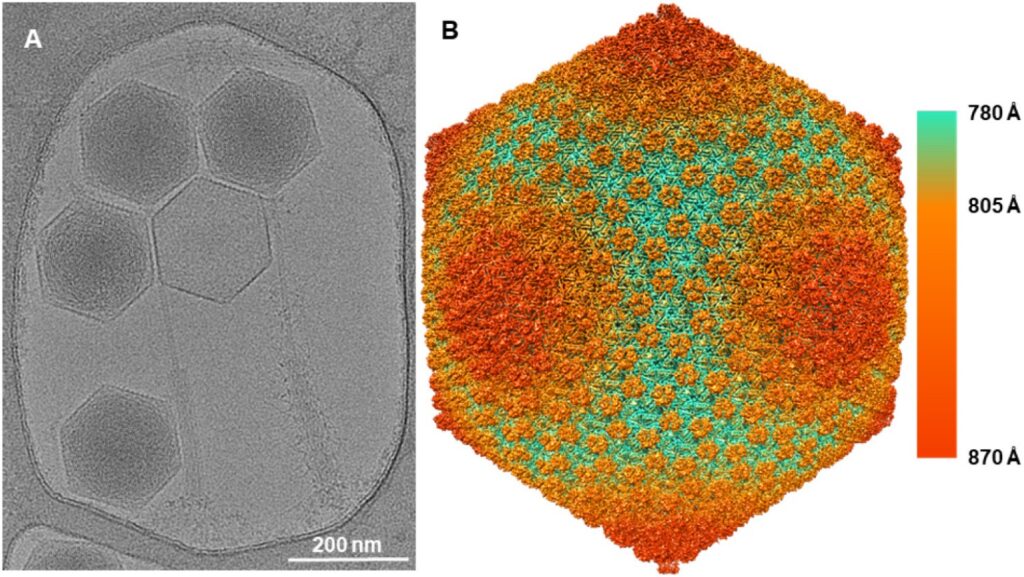
Structural basis of Norovirus assembly and host cell entry
Human noroviruses (HuNoVs) are the major cause of non-bacterial gastroenteritis worldwide. Despite the serious public health concern, information about the infection mechanism and pathogenesis of HuNoVs is limited and there is no vaccine or antiviral. Together with our collaborators, we have established the primate Tulane virus (TV) as a model system for HuNoVs studies. We use TV and HuNoV viral particles to understand the structural basis of viral assembly and the interactions between HuNoVs and their cellular receptors. We also study the attachment/penetration of viral paritcles into host cells, the conformational dynamics of the viral capsid, and genome release in the early stage of viral infection. We also aim to develop the TV system into a surrogate for antiviral screening and evaluation. We use cryo-EM and cryo-ET to determine near-atomic resolution (2-4 Å) structures of TV, the dynamic conformations accompanying receptor binding and genome release, and its complex with antiviral compounds.
Bacteriophage structures and applications
By understanding the phage structure, from host-attachment to virion assembly, we can better understand how structure influences function. Phages are the most abundant life form on the planet, and we know surprisingly little about phage diversity. Together with our collaborators, we are studying the infection process and using cryo-EM to determine the structure of large, tailed phages. We are also interested in developing phage capsids as drug delivery vehicles.
Affinity Cryo-EM: solving macromolecular structures from single-cells
We have previously developed cryo-EM grids with on-grid affinity binding capability (such as Ni-NTA, antibodies, and covalent conjugation) to overcome low yield and short-lived unstable samples. We are refining the method to demonstrate 2-4 Å resolution capability of the affinity grid method for smaller, lower symmetry protein complexes not only for purified, high concentration samples but also for direct capture of target macromolecules from cell lysates or virus particles from patient samples to solve native state structures. We are also interested in applying affinity cryo-EM to study drug-target complexes in cell lysates to extend structure based drug design from an artificial chemical buffer environment to a native cellular environment with the aim to discover more relevant structural information and improve the success rate of drug discovery.
CryoVR: virtual reality augmented hands-on training for cryo-EM
The transmission electron microscope is a complex instrument that requires tedious manual operations with a steep learning curve. Together with collaborators, we are developing a virtual reality-augmented training system called CryoVR which allows new users to learn and practice cryoEM procedures at their own pace and quickly master the operation skills without the fear of damaging expensive equipment or being delayed by the limited availability of trainers and instruments. The CryoVR training system will help reduce accidental damage to the expensive microscopes and sample preparation instruments by novice users, reduce downtime, and maximize the service capacity of the instruments.
Follow Us